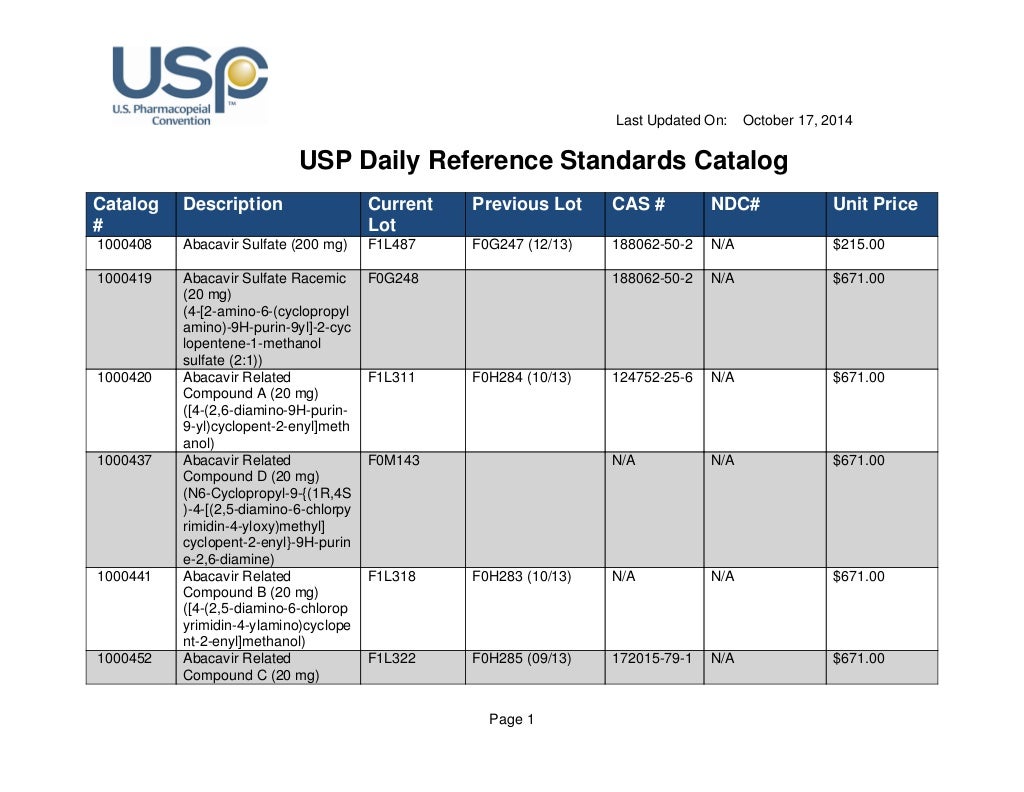
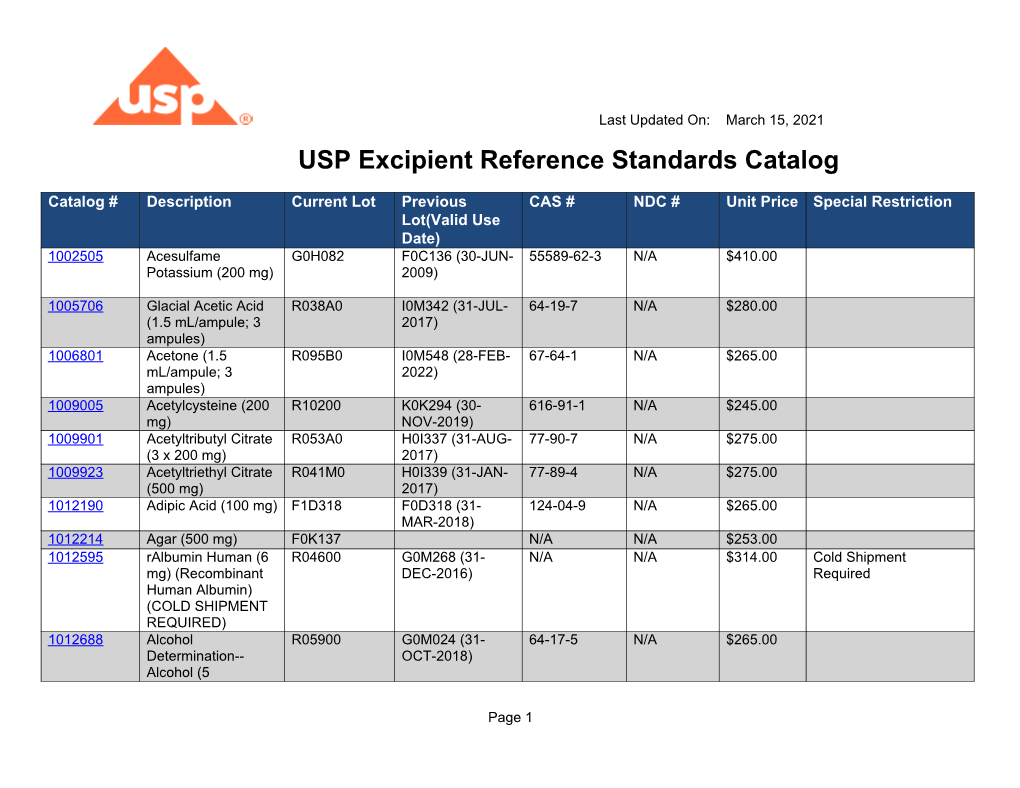
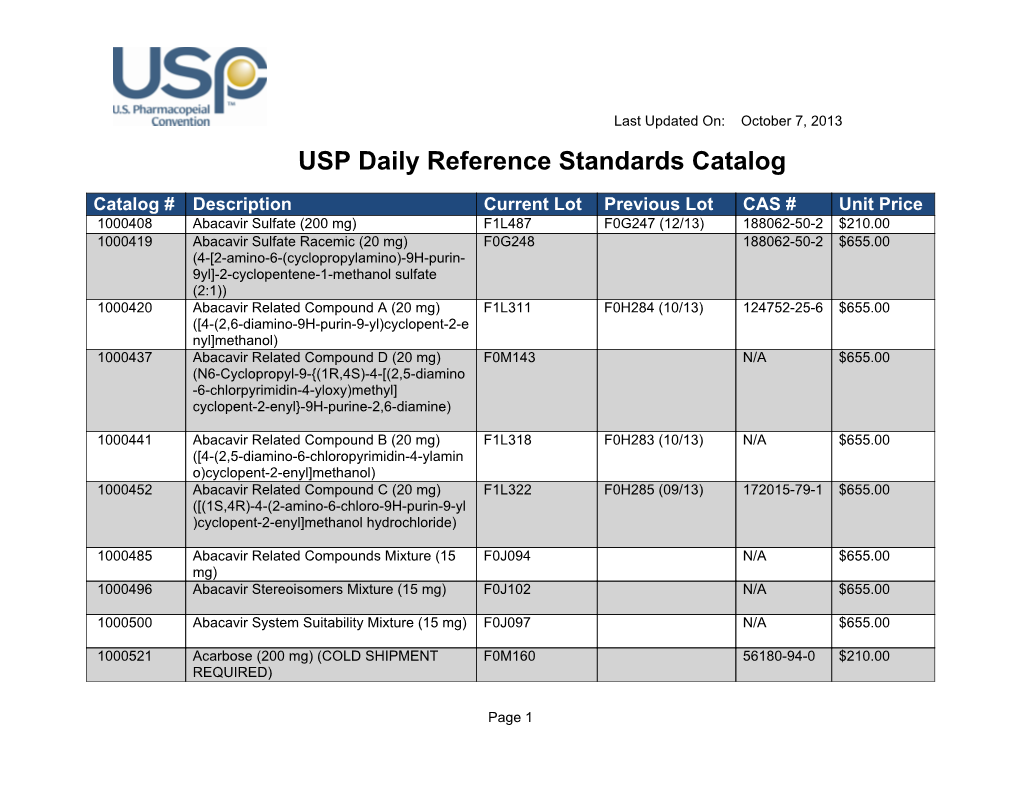
Usp Reference Standard Catalogue
Usp Reference Standard Catalogue - You can also download the european pharmacopoeia daily reference standards catalogue: Search by reference standard name, catalog number, or cas number. Usp offers more than 3,500 highly characterized specimens of drug substances, excipients, food ingredients, and other compounds for analytical testing. Learn how usp develops and releases reference standards for analytical, clinical, pharmaceutical, and research laboratories. Below are several tables with the latest usp reference standard (rs) information for the month of may (2020). See the list of newly available and unavailable rs as of april 28,. The catalog covers a wide range of ingredients,. Learn about the uses, development, availability and validity of usp reference standards, which are intended for test and assay use only. Find and buy usp reference standards for testing drug substances, excipients, food ingredients and dietary supplements. The official catalog of reference standards and authentic. Find out how to order, prepare and use usp rs's. The catalog covers a wide range of ingredients,. Find and buy usp reference standards for testing drug substances, excipients, food ingredients and dietary supplements. Find the latest information on usp reference standards (rs) availability, validity, and changes for the month of june 2020. See the tables for new, existing, and backordered rs lots, and the. In a xml format please note that you can download the terms and conditions of. Live microbial products (lmps) can exist in multiple regulatory categories, as drugs/biologics (live biotherapeutic products), dietary supplements and foods (commonly called probiotics), and. Learn about usp, its documentary and physical standards, and how. Below are several tables with the latest usp reference standard (rs) information for the month of may (2020). Browse the quality solution sheets (qss) to access all the relevant. Find the latest information on usp reference standards (rs) availability, validity, and changes for the month of june 2020. Usp offers more than 3,500 highly characterized specimens of drug substances, excipients, food ingredients, and other compounds for analytical testing. In a xml format please note that you can download the terms and conditions of. Usp reference standards are substances of. Learn about the uses, development, availability and validity of usp reference standards, which are intended for test and assay use only. To help users of usp reference standards locate and apply materials relevant to their work, usp offers these convenient tools and resources: The catalog covers a wide range of ingredients,. Find the latest information on usp reference standards (rs). Download the catalog, access the. To help users of usp reference standards locate and apply materials relevant to their work, usp offers these convenient tools and resources: Learn how usp reference standards are linked to. The document covers the scope, process,. A pdf document that lists the usp reference standards for dietary supplements, including their names, codes, cas numbers, and. Search by reference standard name, catalog number, or cas number. To help users of usp reference standards locate and apply materials relevant to their work, usp offers these convenient tools and resources: Usp offers over 7,000 highly characterized control samples of drug substances, excipients, food ingredients, and more for quality testing. In a xml format please note that you can. Find the latest information on usp reference standards (rs) availability, validity, and changes for the month of june 2020. Live microbial products (lmps) can exist in multiple regulatory categories, as drugs/biologics (live biotherapeutic products), dietary supplements and foods (commonly called probiotics), and. A pdf document that lists the usp reference standards for dietary supplements, including their names, codes, cas numbers,. Learn how usp develops and releases reference standards for analytical, clinical, pharmaceutical, and research laboratories. Learn how usp reference standards are linked to. Usp offers more than 3,500 highly characterized specimens of drug substances, excipients, food ingredients, and other compounds for analytical testing. Live microbial products (lmps) can exist in multiple regulatory categories, as drugs/biologics (live biotherapeutic products), dietary supplements. The document covers the scope, process,. Download the catalog, access the. Learn about usp, its documentary and physical standards, and how. A pdf document that lists the usp reference standards for dietary supplements, including their names, codes, cas numbers, and prices. Usp reference standards are substances of high purity and critical characteristics used for pharmacopeial assays and tests. See the list of newly available and unavailable rs as of april 28,. Usp offers more than 3,500 highly characterized specimens of drug substances, excipients, food ingredients, and other compounds for analytical testing. Find out how to order, prepare and use usp rs's. Find the latest information on usp reference standards (rs) availability, validity, and changes for the month of. Learn about the uses, development, availability and validity of usp reference standards, which are intended for test and assay use only. Usp offers over 7,000 highly characterized control samples of drug substances, excipients, food ingredients, and more for quality testing. Learn how usp develops and releases reference standards for analytical, clinical, pharmaceutical, and research laboratories. Find out what's new at. Find out how to order, prepare and use usp rs's. This reference standard (rs) supports general chapter dissolution. To help users of usp reference standards locate and apply materials relevant to their work, usp offers these convenient tools and resources: The official catalog of reference standards and authentic. Find and buy usp reference standards for testing drug substances, excipients, food. Search by reference standard name, catalog number, or cas number. See the list of newly available and unavailable rs as of april 28,. To help users of usp reference standards locate and apply materials relevant to their work, usp offers these convenient tools and resources: Find out how to order, prepare and use usp rs's. In a xml format please note that you can download the terms and conditions of. Find the latest information on usp reference standards (rs) availability, validity, and changes for the month of june 2020. Find and buy usp reference standards for testing drug substances, excipients, food ingredients and dietary supplements. This reference standard (rs) supports general chapter dissolution. The official catalog of reference standards and authentic. The catalog covers a wide range of ingredients,. Learn how usp develops and releases reference standards for analytical, clinical, pharmaceutical, and research laboratories. Live microbial products (lmps) can exist in multiple regulatory categories, as drugs/biologics (live biotherapeutic products), dietary supplements and foods (commonly called probiotics), and. Usp offers over 7,000 highly characterized control samples of drug substances, excipients, food ingredients, and more for quality testing. Usp offers more than 3,500 highly characterized specimens of drug substances, excipients, food ingredients, and other compounds for analytical testing. Download the catalog, access the. The document covers the scope, process,.Usp daily reference standards catalog
Usp daily reference standards catalog
USP Excipient Reference Standards Catalog DocsLib
USP Daily Reference Standards Catalog DocsLib
USP Reference Standards Catalog DocsLib
USP Reference Standards Catalog Last Updated On November 7, 2020
USP Dietary Supplements Reference Standards Catalog PDF Magnesium
11 USP Reference Standards PDF Metrology Calibration
Official Usp Reference Standards PDF Metrology Calibration
USP標準品|日本バリデーション・テクノロジーズ
Individual Sds Can Be Downloaded, Saved, And Printed.
You Can Also Download The European Pharmacopoeia Daily Reference Standards Catalogue:
Find Out What's New At Usp, Including Reference Standards, Monographs, Impurities, And More.
Learn How Usp Reference Standards Are Linked To.
Related Post: